When the Reaction Produces No Intermediates Which of the Following
The maximum ozone concentration occurs at an altitude of about 15 km and is known as the tropopause 4. Reaction that takes place precisely as depicted in its chemical equation intermediate molecule or ion produced in one step of a reaction mechanism and consumed in another molecularity number of reactant species atoms molecules or ions involved in an elementary reaction rate-determining step.

Reaction Of Hexamethyldisilazane With Silica Water Synthesis Silica Reactions
K ES E P k.

. E no correct response. Intermediate reactions are common in the biological world. Consider the following reaction.
A prime example can be seen in the metabolism of metabolites and nutrients. C the condensation reaction that produces citrate. Reaction intermediates are HOOBr and HOBr b Write a balanced overall equation for this reaction.
And thus a reaction intermediate is almost always formed during the course of a reaction. A reaction intermediate is transient species within a multi-step reaction mechanism that is produced in the preceding step and consumed in a subsequent step to ultimately generate the final reaction product. Reaction Intermediates and Mechanisms.
A reaction intermediate is a step within a larger process of a chemical reaction that is necessary for the outcomes but is often overlooked in chemical equations. B high levels of NAD allow the reaction to proceed. The S N 1 reaction we see an example of a reaction intermediate a very important concept in the study of organic reaction mechanisms that was introduced earlier in the module on organic reactivity Recall that many important organic reactions do not occur in a single step.
Chemistry questions and answers. A the reaction catalyzed by succinate dehydrogenase. 2 NOgO2g 2NO2g 2 NO g O 2 g 2 NO 2 g The overall equation suggests that two NO molecules collide with an oxygen molecule forming NO 2.
The addition of elementary steps produces complex non-elementary reactions. Class 5 The Fish Tale Across the Wall Tenths and Hundredths Parts and Whole Can you see the Pattern. Carbanions prefer a lesser degree of alkyl substitution.
C There are two stages each of which involves a series of five reactions. Roughly 90 of the Earths ozone is found in the thermosphere. By definition of elementary reactions they have 0 intermediates because they cannot be broken down.
A ceproducts label1272b The rate equation for a unimolecular reaction is. The overall chemical reaction is the sum of the two elementary steps. When the reaction produces no intermediates which of the following rate constants will be equal to kcat.
An intermediate is the reaction product of each of. In a Reaction Mechanism a Reaction Intermediate is a molecular entity that is formed by the reactants which reacts further to form the products. Rather they are the sum of two or more discreet bond-forming bond-breaking steps and.
B the reaction that produces oxalacetate. In the second step that molecule of N 2 O 2 collides with a molecule of O 2 to produce two molecules of NO 2. In two of the first three steps of glycolysis a phosphorylation reactions occur.
When the reaction produces no intermediates which of the following rate constants will be equal to kcat. It was produced in the first elementary step then reacts in the second elementary step. Here we present a quick guide to Reaction Intermediate hierarchies.
C concentrations of oxaloacetate are kept very low by rapid use in the subsequent step. The first step in both reactions is the same. The ozone cycle in the stratosphere is responsible for the rise in temperature that reaches a maximum at the stratopause.
Which of the following are intermediate of the reaction. It is important to know the hierarchy of Reaction Intermediates such as Radicals Carbocations Carbanions. The ceNO3 molecule is an intermediate in the reaction a species that does not appear in the balanced chemical equation for the overall reaction.
A an elevated H allows the reaction to proceed. It is a unimolecular reaction and that accounts for the 1 in the names S_N1 and E1. Again by definition of an elementary reaction a single-step reaction will have 1 transition state.
This is the rate determining step. Radicals and Carbocations prefer a greater degree of alkyl substitution. The N 2 O 2 molecule is not part of the overall reaction.
The following mechanism has been proposed for reaction of HBr with O 2 to form H 2 O and Br 2 HBr O 2 HOOBr HOOBr HBr 2 HOBr HOBr HBr H 2 O Br 2 a Identify all reaction intermediates in this reaction. Departure of the leaving group to form an intermediate carbocation. View solution View more.
While Carbanions are the opposite. D more than one correct response. However because termolecular reactions are extremely rare this reaction most likely consists of two or more elementary steps.
Which of the following reaction does not involve A as an intermediate. D the reaction catalyzed by α-ketoglutarate dehydrogenase. Unimolecular Elementary Reactions.
B All reactions take place in the cytosol of a cell. S_N1 Reaction If the nucleophile attacks and bonds to the cationic. Both S_N1 and E1 reactions feature carbocation intermediates.
D the enzyme is unique in its ability only to catalyze the reaction in one direction. The correct statements are a and e. Summing steps 1 and 2 and canceling on both sides of the equation gives the overall balanced chemical equation for the reaction.
You may have to. The molecularity of an elementary reaction is the number of reactant species atoms molecules or ions. C k1 NAD d k2.
Ky k2 E S ES E P ki a k b k2 E harc k. CLASSES AND TRENDING CHAPTER. Learn the specific types of.
In chemistry a reaction intermediate or an intermediate is a molecular entity that is formed from the reactants or preceding intermediates and reacts further to give the directly observed products of a chemical reactionMost chemical reactions are stepwise that is they take more than one elementary step to complete. For example a unimolecular reaction involves the rearrangement of a single reactant species to produce one or more molecules of product. It is formed as a product of the first step but is consumed in the second.
NAD d None of those View Theory. Which of the following steps of the citric acid cycle conserves the energy of a high energy thioester bond. Most chemical reactions require more than one step to form the products.
E S k a None of those b k.
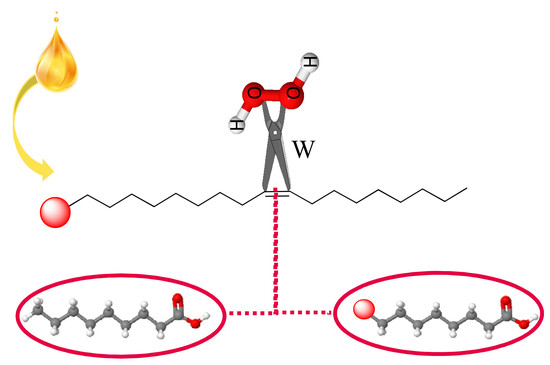
Catalysts Free Full Text Oxidative Cleavage Of Fatty Acid Derivatives For Monomer Synthesis Html

Biochemistry Infographic Breaking Down Triglycerides Biochemistry Chemistry Organic Chemistry

Tris 2 2 Bipyridyl Ruthenium Ii Nanomaterial Co Reactant Electrochemiluminescence Chen 2019 Chemelectrochem Wiley Online Library
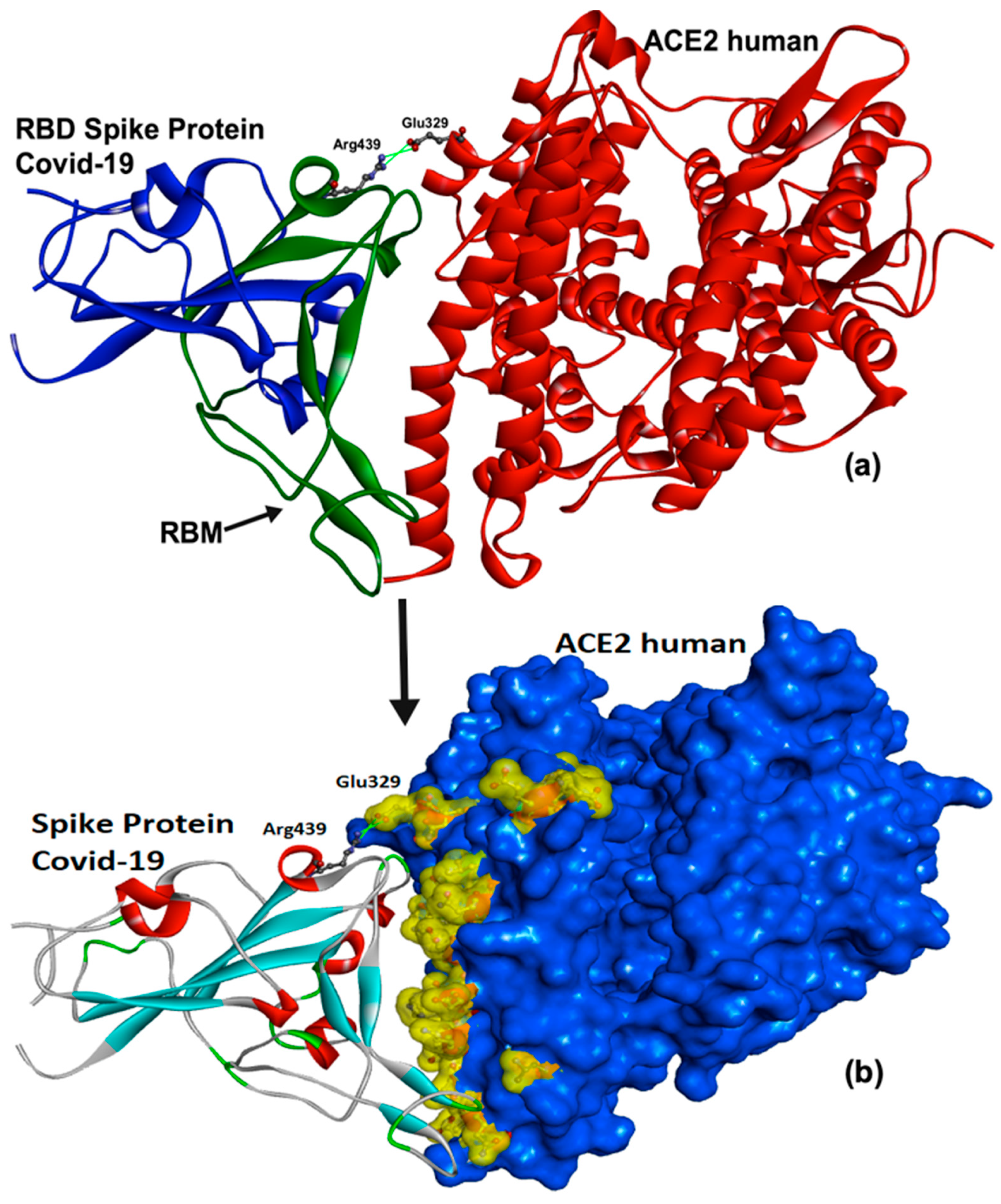
Molecules Free Full Text Natural Flavonoids As Potential Angiotensin Converting Enzyme 2 Inhibitors For Anti Sars Cov 2 Html

Unlocking The Potential Of Substrate Directed Co2 Activation And Conversion Pushing The Boundaries Of Catalytic Cyclic Carbonate And Carbamate Formation Limburg 2020 Chemsuschem Wiley Online Library
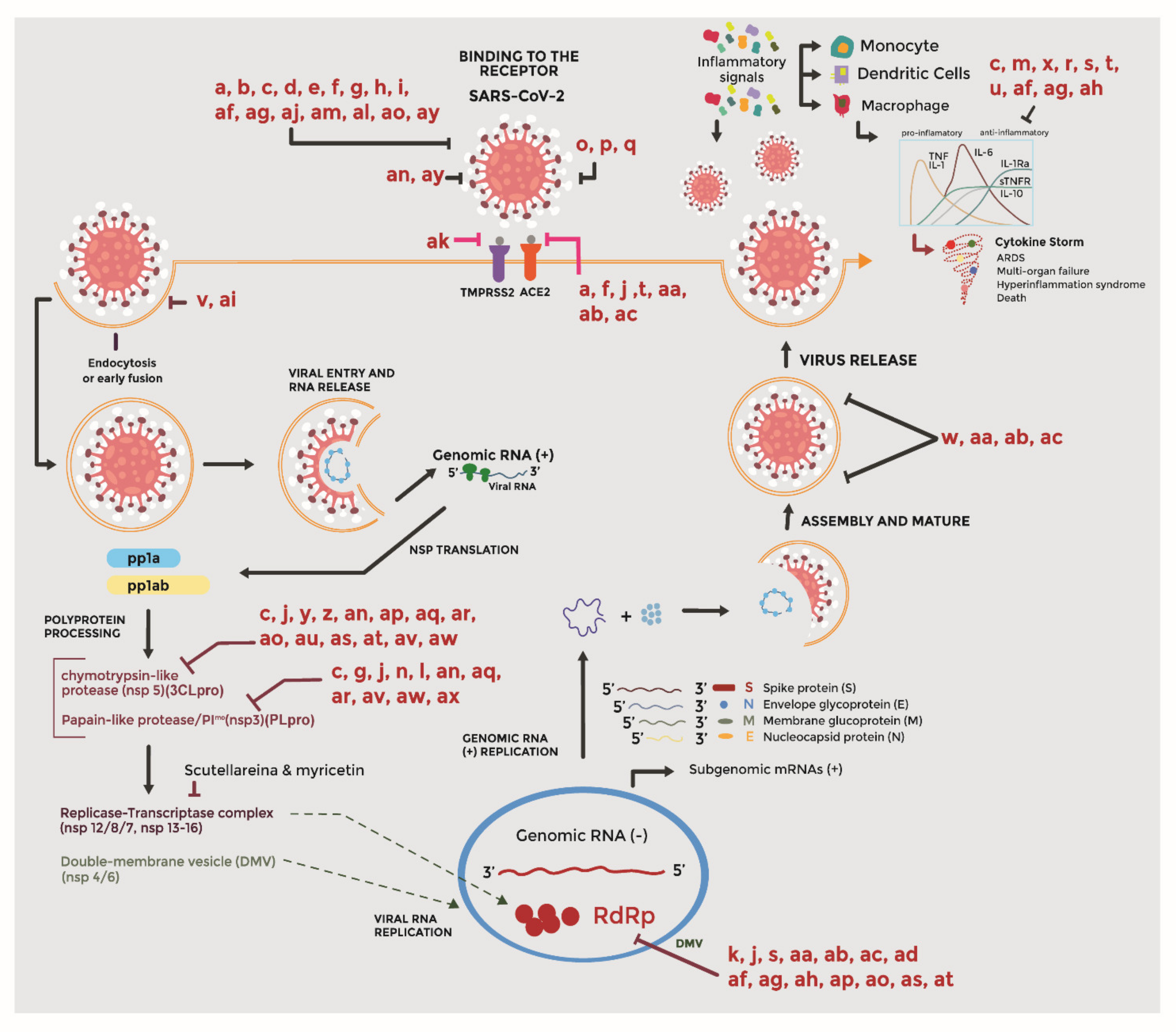
Molecules Free Full Text Plants And Natural Products With Activity Against Various Types Of Coronaviruses A Review With Focus On Sars Cov 2 Html

Molecules Free Full Text Development And Application Of Liquid Crystals As Stimuli Responsive Sensors Html

Titanosilicate Epoxidation Catalysts A Review Of Challenges And Opportunities Smeets 2022 Chemcatchem Wiley Online Library

Tj Oxidative Phosphorylation The Actual Synthesis Of Atp Resulting From Energy Transfer Of The Ets Oxidative Phosphorylation Potential Energy Energy Transfer
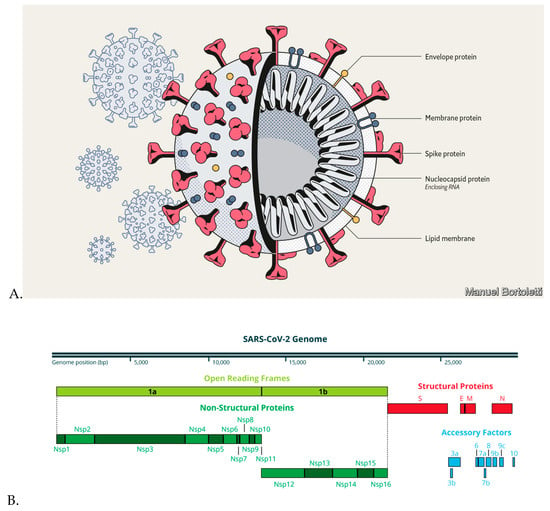
Molecules Free Full Text Natural Flavonoids As Potential Angiotensin Converting Enzyme 2 Inhibitors For Anti Sars Cov 2 Html

Organics Free Full Text On The Question Of Zwitterionic Intermediates In The 3 2 Cycloaddition Reactions A Critical Review Html
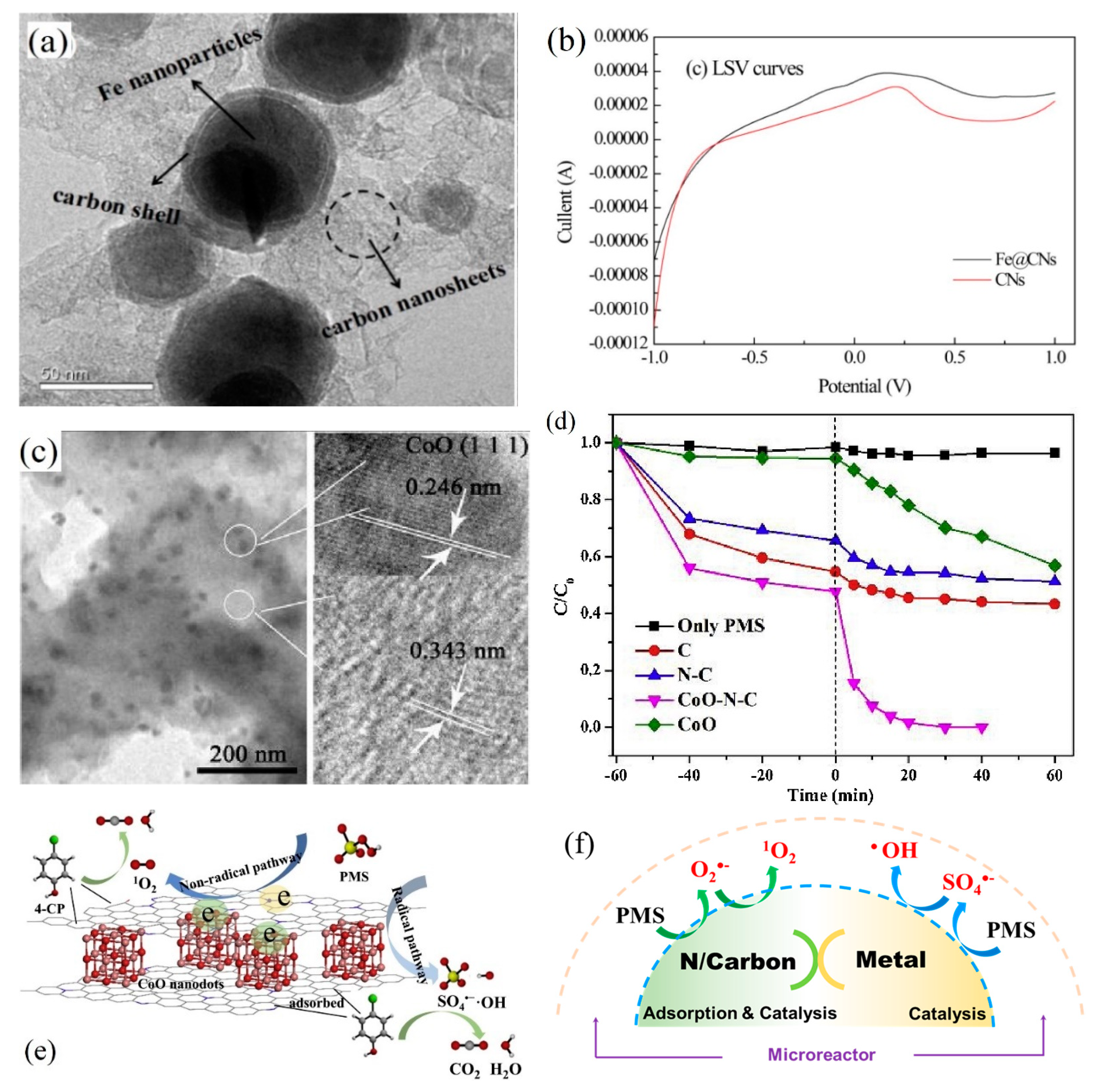
Ijerph Free Full Text Evolution Of Singlet Oxygen By Activating Peroxydisulfate And Peroxymonosulfate A Review Html

An Overview Of Recent Advances In The Applications Of Click Chemistry In The Synthesis Of Bioconjugates With Anticancer Activities Mashayekh 2019 Chemistryselect Wiley Online Library

Progress In Photoinduced Radical Reactions Using Electron Donor Acceptor Complexes Zheng 2021 Asian Journal Of Organic Chemistry Wiley Online Library

Reaction Of Hexamethyldisilazane With Silica Water Synthesis Silica Reactions

Unlocking The Potential Of Substrate Directed Co2 Activation And Conversion Pushing The Boundaries Of Catalytic Cyclic Carbonate And Carbamate Formation Limburg 2020 Chemsuschem Wiley Online Library

Electrochemical Carbon Dioxide Reduction At Nanostructured Gold Copper And Alloy Materials Vickers 2017 Energy Technology Wiley Online Library

Wolff Rearrangement Of Oxidatively Generated A Oxo Gold Carbenes An Effective Approach To Silylketenes Zheng 2019 Angewandte Chemie Wiley Online Library

Comments
Post a Comment